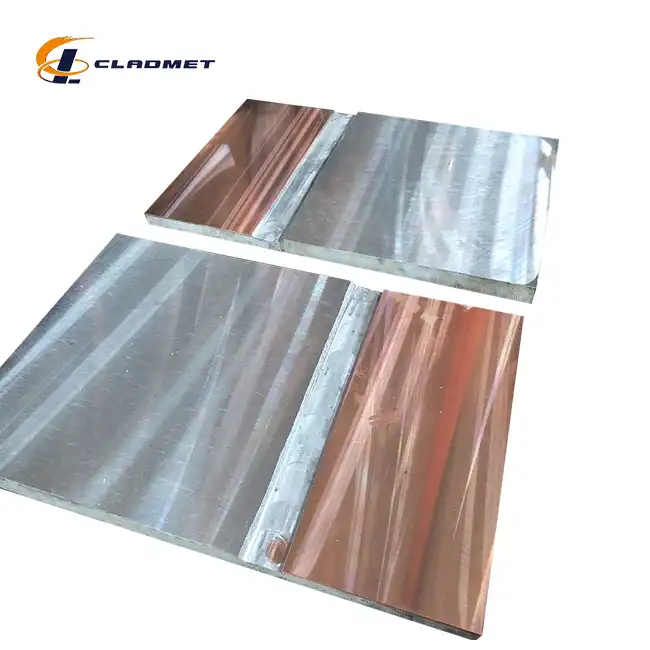
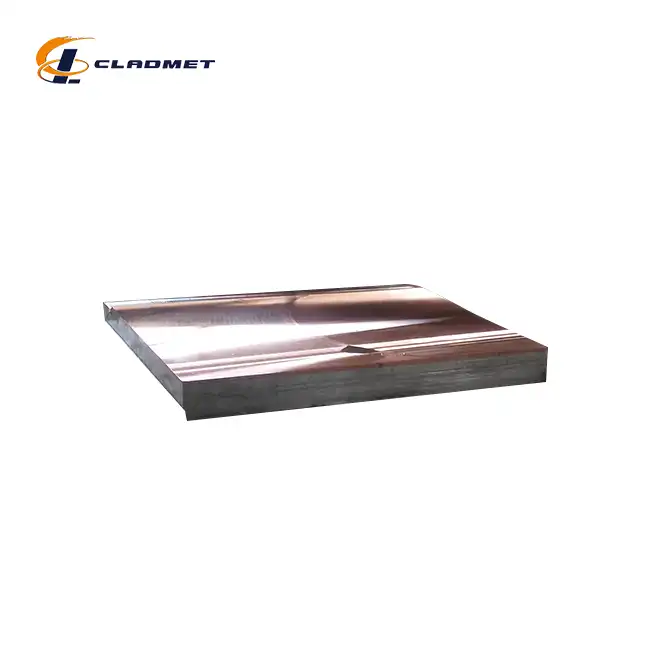
Gr12 Titanium Clad S31608 Stainless Steel Plate for Pharmaceutical Use absolutely meets sterile standards required in pharmaceutical manufacturing environments. This advanced composite material combines the exceptional corrosion resistance and biocompatibility of Grade 12 titanium with the mechanical strength and cost-effectiveness of S31608 stainless steel core. The titanium cladding provides superior resistance to aggressive pharmaceutical chemicals, steam sterilization, and cleaning agents while maintaining the structural integrity needed for demanding applications. These plates comply with FDA regulations, USP standards, and ISO requirements for pharmaceutical equipment materials, making them ideal for sterile processing environments where contamination prevention is paramount.
Understanding the Properties of Gr12 Titanium Clad S31608 Stainless Steel Plates
The unique construction of these clad plates creates an optimal balance between performance and practicality. The S31608 stainless steel core delivers excellent mechanical properties, including high tensile strength and ductility, while the Grade 12 titanium outer layer provides unmatched chemical resistance and biocompatibility essential for pharmaceutical applications.
Chemical Composition and Structure
Grade 12 titanium contains small amounts of nickel and molybdenum, enhancing its corrosion resistance compared to commercially pure titanium grades. The S31608 stainless steel core features chromium, nickel, and molybdenum content that provides structural stability and cost efficiency. This combination creates a synergistic effect where each material compensates for the limitations of the other, resulting in superior performance characteristics.
Enhanced Corrosion Resistance
Pharmaceutical environments expose materials to aggressive cleaning chemicals, organic solvents, and acidic compounds. The titanium cladding demonstrates exceptional resistance to chloride-induced corrosion, which commonly affects standard stainless steel in pharmaceutical cleaning protocols. Testing data shows corrosion rates below 0.02 mm per year under accelerated pharmaceutical simulation conditions, significantly outperforming conventional materials.
Biocompatibility and Surface Properties
The smooth titanium surface naturally resists bacterial adhesion and biofilm formation, crucial factors in maintaining sterile conditions. Surface roughness typically measures below Ra 0.4 μm, creating an environment where pathogenic microorganisms cannot establish colonies even under challenging conditions.
Sterile Standards and Pharmaceutical Compliance for Titanium Clad Stainless Steel
Pharmaceutical manufacturing demands the highest levels of material compliance with international standards. Regulatory bodies worldwide have established stringent requirements that materials must meet before approval for use in sterile environments.
FDA and USP Requirements
The Food and Drug Administration mandates that pharmaceutical equipment materials demonstrate non-reactivity with drug compounds and resist degradation under sterilization conditions. United States Pharmacopeia standards specify requirements for extractable substances, surface finish, and cleanability. Gr12 titanium clad plates meet these specifications through their inert surface chemistry and resistance to leaching.
Sterilization Method Compatibility
These plates withstand multiple sterilization protocols without material degradation. Steam autoclaving at 121°C for extended cycles causes no structural changes or surface deterioration. Clean-in-Place (CIP) systems using caustic solutions and acids encounter no material resistance from the titanium surface. Sterilize-in-Place (SIP) protocols maintain material integrity through repeated high-temperature exposure cycles.
International Certification Standards
Compliance extends beyond American standards to include European PED certification and Japanese JIS specifications. The material meets ASME and ASTM requirements for pressure vessel applications, ensuring global acceptance in pharmaceutical manufacturing facilities worldwide.
Comparing Gr12 Titanium Clad vs. Standard S31608 Stainless Steel Plates for Pharmaceuticals
Understanding performance differences helps procurement professionals make informed material selection decisions. While standard stainless steel offers lower initial costs, the enhanced properties of titanium cladding provide significant long-term advantages in pharmaceutical applications.
Performance Analysis
Standard S31608 stainless steel shows acceptable performance in many pharmaceutical applications but faces limitations when exposed to chlorinated cleaning agents and certain pharmaceutical compounds. Pitting corrosion can develop over time, creating surface irregularities that harbor contaminants. Titanium cladding eliminates these concerns through superior chemical inertness and uniform corrosion resistance across diverse pharmaceutical environments.
Cost-Benefit Evaluation
Initial investment in titanium clad plates typically exceeds standard stainless steel costs by 40-60%. However, total cost of ownership calculations reveal significant savings through extended service life, reduced maintenance requirements, and decreased downtime for cleaning and inspection. Pharmaceutical facilities report 3-5 times longer service intervals between major equipment refurbishment when using titanium clad materials.
Alternative Material Considerations
Duplex stainless steels offer improved chloride resistance compared to standard grades but cannot match titanium's biocompatibility and chemical inertness. Higher titanium grades provide enhanced properties but increase costs without proportional benefits for most pharmaceutical applications. The Gr12 titanium clad configuration optimizes performance while maintaining economic viability.
Procurement Insights: How to Source Gr12 Titanium Clad S31608 Stainless Steel Plates
Successful procurement requires careful supplier evaluation and specification development. The specialized nature of these materials demands attention to manufacturing capabilities, quality systems, and certification documentation.
Supplier Qualification Criteria
Manufacturers must demonstrate explosive bonding expertise and quality control systems that ensure consistent cladding integrity. ISO 9001 certification represents minimum requirements, while additional pharmaceutical-specific certifications enhance supplier credibility. Production capabilities should include metallurgical testing, surface finish control, and dimensional precision meeting pharmaceutical equipment tolerances.
Technical Specifications
Procurement specifications must define plate thickness, surface finish requirements, dimensional tolerances, and mechanical properties. Titanium cladding thickness typically ranges from 1-3mm depending on application severity, while total plate thickness varies based on structural requirements. Surface finish specifications should include roughness values, cleaning validation requirements, and visual appearance standards.
Documentation and Traceability
Material certificates must include chemical composition verification, mechanical property testing, and bond strength confirmation. Traceability documentation should track raw materials through manufacturing processes, enabling full accountability in pharmaceutical validation protocols. Third-party inspection reports provide additional assurance of compliance with specified requirements.
Installation, Maintenance, and Technical Support for Pharmaceutical Use
Proper implementation ensures optimal performance throughout the service life. Installation techniques and maintenance protocols significantly impact material performance in pharmaceutical environments.
Installation Best Practices
Welding procedures require specialized techniques that preserve the titanium cladding integrity while maintaining structural strength. Heat input control prevents excessive temperature exposure that could compromise the clad interface. Post-weld heat treatment may be necessary depending on application requirements and stress relief needs.
Maintenance Protocols
Regular inspection schedules should include visual examination for surface irregularities, cleaning effectiveness validation, and periodic corrosion monitoring. Cleaning procedures must use pharmaceutical-grade agents that maintain surface integrity while achieving sterility requirements. Documentation protocols track cleaning cycles, inspection results, and performance trends over time.
Technical Support Requirements
Ongoing technical support ensures optimal performance throughout the equipment lifecycle. Expert consultation helps resolve application-specific challenges and optimize cleaning protocols for maximum effectiveness. Training programs educate facility personnel on proper handling, maintenance, and troubleshooting procedures.
JL CLAD METALS: Your Trusted Gr12 Titanium Clad S31608 Stainless Steel Plate Manufacturer
Baoji JL Clad Metals Materials Co., Ltd. specializes in manufacturing premium Gr12 titanium clad S31608 stainless steel plates specifically engineered for pharmaceutical applications. Our explosive composite technology ensures superior bonding strength and uniform cladding thickness across large plate dimensions.
Advanced Manufacturing Capabilities
Our independent explosive composite technology creates metallurgical bonds that exceed industry standards for strength and durability. Self-rolling plate production capabilities enable custom thickness combinations and surface finishes tailored to specific pharmaceutical requirements. Quality control systems include ultrasonic bond testing, surface roughness measurement, and comprehensive chemical analysis.
International Certifications and Standards
JL CLAD METALS maintains ISO9001-2000 certification and recently achieved PED and ABS international qualifications in 2024. Our manufacturing processes strictly follow GB/GBT, ASME/ASTM, and JIS standards, ensuring global compliance and acceptance. Continuous improvement programs keep our capabilities aligned with evolving pharmaceutical industry requirements.
Customization and OEM Services
We offer comprehensive OEM services including precision cutting, custom surface treatments, and specialized certification support. Our technical team collaborates with clients to develop optimized solutions for unique pharmaceutical applications. Research and development capabilities enable innovative design solutions that address emerging challenges in pharmaceutical manufacturing.
Conclusion
Gr12 Titanium Clad S31608 Stainless Steel Plate for Pharmaceutical Use definitively meets sterile standards required in pharmaceutical manufacturing. The unique combination of titanium's biocompatibility and corrosion resistance with stainless steel's mechanical strength creates an optimal material solution for demanding pharmaceutical environments. Compliance with international standards, superior performance characteristics, and cost-effective lifecycle economics make these plates the preferred choice for sterile pharmaceutical applications. JL CLAD METALS' expertise in explosive composite technology and commitment to quality ensures reliable supply of materials that exceed pharmaceutical industry expectations.
Frequently Asked Questions
Q1: What specific sterilization temperatures can Gr12 titanium clad plates withstand?
A: These plates withstand steam sterilization temperatures up to 150°C without degradation. The titanium cladding maintains structural integrity and surface properties through repeated autoclaving cycles at 121-134°C, standard pharmaceutical sterilization temperatures.
Q2: How does the bonding strength between titanium and stainless steel affect pharmaceutical applications?
A: Explosive bonding creates metallurgical bonds exceeding 300 MPa shear strength, preventing delamination under pharmaceutical processing conditions. This ensures long-term reliability and eliminates contamination risks from interface failure.
Q3: What documentation is provided for pharmaceutical validation purposes?
A: Complete material certificates include chemical composition verification, mechanical properties, bond strength testing, surface finish measurements, and traceability documentation meeting FDA and international pharmaceutical standards requirements.
Get Premium Gr12 Titanium Clad S31608 Stainless Steel Plates from JL
Ready to enhance your pharmaceutical manufacturing capabilities with superior materials? JL CLAD METALS delivers world-class Gr12 Titanium Clad S31608 Stainless Steel Plate for Pharmaceutical Use backed by cutting-edge explosive composite technology and comprehensive quality assurance. Our proven track record in serving global pharmaceutical manufacturers ensures you receive materials that exceed sterile standards while optimizing operational efficiency. As a leading titanium clad stainless steel plate supplier, we provide customized solutions, expert technical support, and reliable delivery schedules that keep your projects on track. Experience the JL advantage through our innovative manufacturing processes, international certifications, and commitment to pharmaceutical industry excellence. Contact us at sales@cladmet.com for detailed specifications, certifications, and competitive quotations tailored to your specific pharmaceutical applications.
References
1. American Society for Testing and Materials. "Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate." ASTM International, 2023.
2. Food and Drug Administration. "Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics." FDA Center for Drug Evaluation and Research, 2022.
3. International Organization for Standardization. "Medical devices - Quality management systems - Requirements for regulatory purposes." ISO 13485:2016.
4. Pharmaceutical Engineering Society. "Baseline Guide: Commissioning and Qualification of Pharmaceutical Manufacturing Systems." ISPE, 2023.
5. United States Pharmacopeial Convention. "General Chapter <1072> Disinfectants and Antiseptics." USP-NF 2024.
6. European Medicines Agency. "Guideline on the sterilisation of the medicinal product, active substance, excipient and primary container." EMA Committee for Medicinal Products for Human Use, 2023.

_1737007724117.webp)
_1736996330512.webp)







 2025-12-30 11:39:21
2025-12-30 11:39:21
